Understanding COVID & friends
On the symptoms and risks of COVID-19 and other respiratory viral infections
By Levien van Zon
This article isn’t short. For those of you who are of an impatient disposition, I have written a short summary of the main points. And for those of you who do read the whole story and want to know even more, I have added footnotes with sources and more background information.
Several weeks ago, I became ill. Initially I just had a mild headache, which I attributed to drinking a few too many craft beers on a Saturday afternoon. But a day or two later I started feeling feverish and tired. A sore throat came soon after, and I had a runny nose for several days, with occasional sneezing. Normally these symptoms obviously wouldn’t be any case for concern: a cold, perhaps, or a mild case of the flu. But I was in Central America, a warm part of the world where a cold or flu didn’t seem all that common, especially in the dry season. Moreover, COVID-19 was just spreading into the Americas. So when I started feeling a pressure on my chest and experiencing shortness of breath, I started to get somewhat worried. This clearly wasn’t a normal cold, and I had read reports of how symptoms such as these can rapidly deteriorate and possibly lead to death. Also, it didn’t help that the nearest decent hospital was several hours away.
Meanwhile, the media seemed to be focusing very much on the statistics of COVID-19. Initially, reports mostly concerned the number of confirmed cases, but as the pandemic progressed, this shifted to the number of hospitalisations, intensive care beds and of course the rapidly growing number of deaths. I was in a country with only a few confirmed cases of COVID-19, but that statistic wasn’t of any use to me. And frustratingly, it was quite hard to find any reliable and consistent information on the actual symptoms and risks associated with COVID-19. So, as a theoretical biologist with some knowledge on virus dynamics, I started doing some research, and collecting some data to get a better idea of what to expect. I wanted to know how my symptoms compared to those of other people, and what the actual risks could be for me as an individual with possible symptoms of COVID-19.
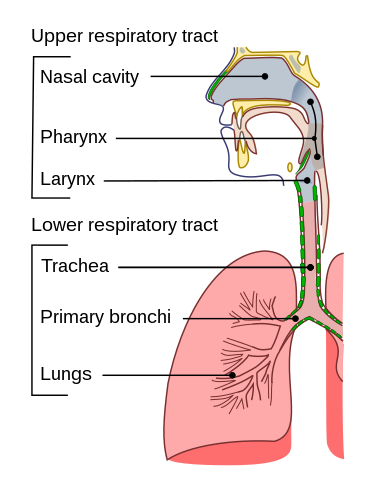
It is actually quite hard to know whether you have the rather creatively named Corona Virus Disease 2019 (COVID-19) or something else, especially in the first few days of symptoms. And the reason for this has to do with the nature of what we call “respiratory infections”. The respiratory system or tract is a fancy term for the parts of our body that we use for breathing: our nose, nasal cavity, throat, lungs and all the bits in between. The upper respiratory tract is roughly everything from the throat upwards, and the lower respiratory tract consists of the bits below that: the windpipe that branches off into the bronchi and further down into an intricate network of spaces in the lungs.
We are all familiar with upper respiratory viral infections. In fact, most adults experience one 2 to 3 times per year, and children a lot more often. The “common cold” is a (rather inaccurate) collective term for an upper respiratory infection, which may be caused by caused by over 200 different types of virus. Roughly half of these are so-called rhinoviruses. Another 10-15% of cases of common cold are caused by human coronaviruses, which have rather obscure names such as HCoV-OC43, and some of which have been circulating in the human population for at least half a century, although probably much longer1.
So what happens if you get a cold? First, you get infected by “live”2 virus particles, also know as virions, either because you inhale tiny droplets that other people produce when sneezing or coughing, or because you pick them up from a surface (or person) that was contaminated with live virions3. Once infectious virions manage to get into your upper respiratory tract, they break inside some of the cells and start instructing them produce new virions, which can then infect neighbouring cells. Oddly, however, this virus replication (the production of new virions) is not what causes the typical symptoms associated with a cold. The symptoms are caused not by the virus itself, but by your immune system. Specialised immune cells can recognise signs of viral and bacterial infections, and trigger the production of “alarm-substances” called cytokines. These cytokines subsequently trigger a whole range of responses in your body. The first effects of cytokine production that you usually notice are the so-called systemic symptoms, which may include fever, headache, muscle ache, loss of appetite and/or malaise, a general feeling of being sick. These systemic symptoms usually develop quite rapidly, but don’t last long, at most a few days. Somewhat more slowly however, cytokines and a number of other substances trigger the process of inflammation at or near the sites where the virus is reproducing itself. This inflammation is actually a rather complicated and useful set of processes that help remove infected and damaged cells, and help initiate tissue repair. Unfortunately we mostly experience this useful process as pain and snot.
The first “local” (rather than systemic) symptom is often irritation of the throat or nasal cavity, which may later develop into a sore throat as inflammation gets worse. Sneezing is another local effect of inflammation, often accompanied by a runny nose. Unfortunately both these responses of your immune system, sneezing and a runny nose, also happen to be great ways of spreading virus particles so that they can infect other people. As the inflammation spreads down in the direction of the lower respiratory tract, it may over-sensitize the nerves that trigger the coughing response, which may then cause you to start coughing at even the slightest irritation of the airways (such as inhaling cold air).5 This is known as an unproductive cough or dry cough (as opposed to a productive or wet cough that actually removes material from your lower airways).
As it turns out, the symptoms I just described aren’t just symptoms of common cold, but of upper respiratory infections in general. Because the symptoms are caused by the immune system rather than a virus, they are more or less the same for the more than 200 viruses that can cause upper respiratory infections in humans. Most of these infections we classify either as common cold or as the flu. Flu or influenza is actually an interesting case, because in contrast to common cold it is an infection caused by a single type of virus, known as Influenza A. Originally a virus of water birds, notably ducks, influenza has also been infecting humans and other mammals for millennia. The resulting illness, called the flu in English and influenza, gripe, Grippe, griep or something similar in most other European languages, is often confused with common cold, but there are a few important differences. It spreads from person to person in more or less the same way as common cold: through coughing or sneezing and through other direct or indirect contact with virions. Due to the way it spreads, the flu generally starts off as an upper respiratory infection, similar to common cold. But while the rhinoviruses that cause many cases of common cold hardly seem to damage the tissues lining the upper respiratory tract, influenza infections can cause tissue damage. Moreover, it seems that an influenza infection can spread to the lower respiratory tract (windpipe, bronchi and lungs) more easily than is common for most common cold viruses. Finally, influenza viruses have a trick up their sleeve called reassortment, which allows them to “shape shift” more easily than other respiratory viruses. This means that they can more easily evade the antibody response that constitutes the fast part of your immune system memory.
As a result of the typical properties of flu viruses, an influenza infection may evoke a stronger immune response than a common cold infection, and has a larger probability of causing complications.4 For instance, fever is uncommon with common cold in adults, but it is reported in roughly 35% of experimental infections with influenza (in adults younger than 65).6 Also other systemic symptoms such as muscle ache and lack of appetite tend to be more common and/or stronger in influenza than in common cold, which suggests a stronger cytokine response, which in turn can be the result of a higher virus load (a larger number of virions) and/or more damaged cells. Generally, adults recover from the flu within 5 to 8 days, but especially in elderly people, young children or people with underlying health problems, potentially dangerous complications can result from an influenza infection. If the virus moves into the lungs, for instance because it is not effectively contained by the immune system, it may cause viral pneumonia. Pneumonia is an inflammation of the lungs, so it is yet another case of symptoms being caused (at least in part) by the immune system rather than a disease agent (virus, bacterium, etc.). The immune reaction in itself is a good thing of course, but if an inflammation of the lungs gets out of hand, it reduces the capacity of the lungs to transfer oxygen to your bloodstream.7 Several factors can aggravate this problem, including lung damage or existing issues with your immune system. A common complication with influenza is that bacteria infect the lung tissue that has been damaged by the virus, causing yet more damage and a stronger local inflammation. Before such secondary infections could be treated by antibiotics they were one of the main causes of human deaths, and in many developing countries they still are. In fact, globally a quarter to half a million people are estimated to die every year due to complications of a flu infection, mainly pneumonia. Elderly people are strongly overrepresented in these deaths, because our immune system tends to react less effectively to infections as we get older. Immune responses get both less strong and slower as people age, which explains why symptoms such as fever tend to be less common or less strong in elderly people. Unfortunately this means that viruses and bacteria are also less effectively contained early on in an infection, allowing them to spread more easily, and eventually leading to more serious problems later on.
So virus infections can turn more serious if your immune system responds slower or less strong early on in the infection. This happens more often in old people, very young people, people with immune system issues, but also for instance in people who are sleep deprived or severely stressed. Moreover, if a virus is not recognised by your immune system, your immune response will initially be less effective at containing it, and the symptoms may eventually be worse. In fact, this is exactly what is thought to happen in pandemics. For instance, the 20th century saw three significant influenza pandemics, with an estimated death toll of respectively 50-100 million people (up 3-6% of the world population!) in 1918, 1-4 million people in 1956 and around 1 million people in 1968. All three pandemics are thought to result from a human influenza virus acquiring a new “coat” from their cousins that infect birds (Avian influenza viruses). The resulting influenza strains were new to our immune systems, and so spread rapidly in the human population and caused more damage than usual.8
And this finally brings us back to COVID-19. Coronaviruses aren’t new to humans, but like influenza they aren’t primarily a human virus. In the same way that influenza is native to ducks, coronaviruses are thought to be native to bats, which support a large diversity of viruses because (like humans) they are very social animals. Occasionally one of these viruses will jump to humans, often through an intermediate animal, as happened in 2003 with the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) in Asia. It happened again in 2012 with the Middle Eastern Respiratory Syndrome coronavirus (MERS-CoV), and most recently in 2019 with a virus we now call SARS-CoV-2. SARS-CoV-2 is the virus that causes COVID-19, and as its name suggests it is very closely related to the 2003 SARS coronavirus. Both viruses grab on to a molecule called ACE2, that is present on the outside of various types of human cells, including lung cells. However, there is an important difference between the 2003 virus that caused SARS and the 2019 virus that causes COVID-19. The older SARS-CoV primarily infected the lower respiratory tract, because lung cells have a lot of ACE2-molecules on their surface, while its younger brother SARS-CoV-2 seems less selective and is much better able to infect the upper respiratory tract, which has fewer ACE2-molecules. This means that while SARS was primarily a lung disease that spread relatively slowly, COVID-19 is both an upper respiratory disease and a lower respiratory disease, and spreads much faster. And this may turn out to be an advantage, almost certainly for the virus and, counter-intuitively, possibly also for us if we’re infected by the virus.
So, what happens if someone in your vicinity has COVID-19 and you’re exposed to live virions of SARS-CoV-2? This virus basically spreads from person to person in the same way as common cold and influenza, so initially it manifests as an upper respiratory infection, with the symptoms we already discussed. Like the other viruses, it triggers cytokine production and an inflammation of the upper respiratory tract. So the first symptoms of COVID-19 you’d experience are the systemic ones (fever, headache, muscle ache, loss of appetite, loss of smell and/or taste9, feeling tired, generally feeling sick), followed somewhat later by local symptoms such as throat ache, runny nose, sneezing and cough. In the second week of March, two hospitals in The Netherlands tested 1353 health care workers with symptoms of respiratory infection, and found 86 (6.4%) to be infected with SARS-CoV-2.10 Of these 86 infected people, 65% reported fever or feeling feverish, 57% reported headache, 17% loss of appetite, 64% muscle ache and 76% reported “general malaise”. In other words, a majority of people with symptoms experienced some form of systemic symptoms, which is more than is generally the case for common cold or flu. As for local symptoms, 40% reported a sore throat, 55% a runny nose and 76% reported coughing. The first two symptoms indicate an upper respiratory infection (unfortunately the percentage of sneezing wasn’t reported), while the high percentage of coughing suggests that the virus may spread downward into the lower respiratory tract (as can also happen with influenza). In fact, 38% of the infected people with symptoms reported shortness of breath, and 29% reported chest pain, both of which may indicate that the virus may have spread into the lungs and caused (mild) viral pneumonia.
Of the 86 health workers who were identified with COVID-19 in the above study, 84 experienced relatively mild symptoms, and two were admitted to hospital, although their situation wasn’t critical. Unfortunately this study only tested people who reported symptoms, so it probably missed health care workers that were infected with SARS-CoV-2 but did not develop any symptoms. This may be more common than you would expect: of the people experimentally infected with influenza, a third did not develop any flu symptoms, and a large multi-year study of British households found that “in the field” the large majority (75%) of flu cases turned out to be asymptomatic (without symptoms). For COVID-19 the percentage of asymptomatic cases isn’t exactly known at the moment, but two early Japanese studies estimated 18-40% and 33-46% (the large range is due to the uncertainty in the incubation period of COVID-19, which is the time between infection and the onset of symptoms).
So what does all this mean for the risks of spreading COVID-19, and the risk of potential life-threatening complications? In order understand that better, let’s take a look at how the virus load (the amount of virus in the body) develops after the onset of symptoms, for influenza, COVID-19 and SARS:11
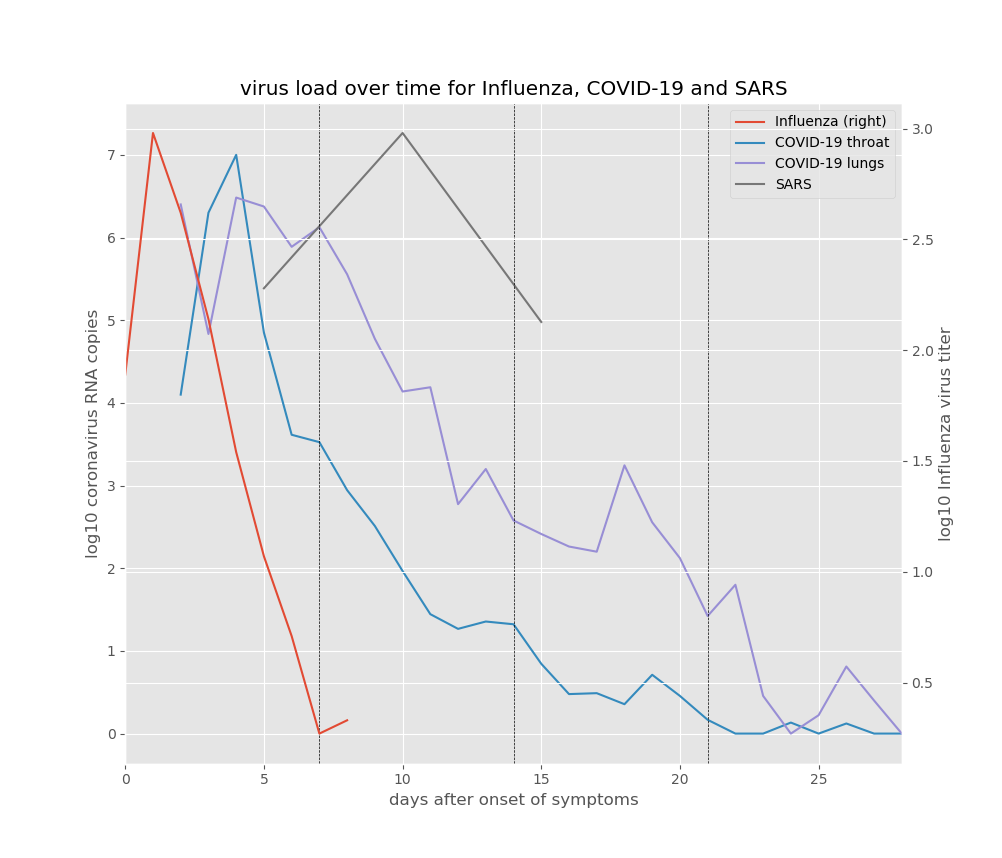
The first thing to notice is that influenza develops and disappears much faster than the two coronaviruses. The influenza virus load tends to peak 1 or 2 days after the onset of symptoms, and then declines rapidly. In most of the adults for which the average influenza virus load is shown, the symptoms were resolved and the virus load had become negligible after five days, although for some it took longer. Influenza is a fairly fast “hit and run” virus: it is generally well adapted to replicating in humans, and on average the time between being infected and infecting another person is around 2.5 days, which makes sense because the risk of infecting another person tends to be highest around the peak in virus load, the time when you’re producing and spreading the most virions. After this peak, the virus load starts to decline as the virus starts running out of cells that can be easily infected, and because your cytokines have kicked your various immune responses into action, which get to work cleaning up infected cells and virions.
While the two corona brothers SARS and COVID may be infamous, they don’t seem particularly fast. Possibly this is because both were still fairly new to the game of infecting humans. While influenza tends to have an incubation period of 1 day in experiments (and up to a few days in real-life settings), COVID-19 is reported to have an average incubation period of 2-6 days, with much longer incubation times (up to two weeks) reported for some cases.12 So while influenza manifests itself quite rapidly, it may take COVID-19 a few days to reach high virus loads and trigger significant cytokine production. However, once the symptoms are there, the peak of virus production in the upper respiratory tract seems to be reached quite rapidly, after about 3 days, after which it declines rapidly, similar to influenza. In fact, after 5 days, no active virus replication was measured in the upper respiratory tract of the 9 patients that were sampled in the study by Wölfel et al. (upon which the COVID-lines in the figure are based), and no live virions could be extracted after 8 days. This means that anyone with COVID-19 will probably be most infectious in the first week of symptoms. The runny nose and sneezing and coughing that often occur in this period help spread virus particles to people and surfaces around you, but once that stops, the risk of infecting others may drop quite rapidly – as is also the case with common cold and flu.
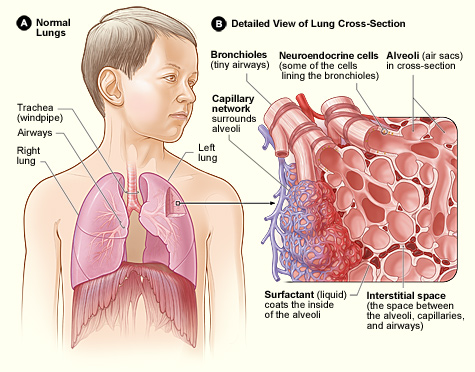
However, as we discussed, COVID-19 isn’t just an upper respiratory infection, and the risk of complications mainly pops up when the SARS brothers spread deeper into the lungs. The virus load in the lungs increases somewhat more slowly, and for COVID-19 it seems to reach a peak around day 5-6 of symptoms. Other sources have reported a peak in hospitalisation around day 6 of symptoms, which would indeed be consistent with the peak in lung virus load observed here. SARS, while similar in its complications, seems to be slower and more deadly than COVID-19. A study in 2003 followed 75 patients with SARS, and found two peaks in serious complications, one around day 11 and one around day 20. The first peak coincides more or less with the peak in lung virus load for SARS. In hospitalised cases of COVID-19, there often seems to be a worsening of symptoms around day 9, slightly earlier than for SARS. A “serious complication” in the case of both SARS and COVID-19 can be the progression into ARDS, acute respiratory distress syndrome. In ARDS, pneumonia gets worse to a point at which your lungs can no longer supply sufficient oxygen for your body to function, and you need mechanical ventilation. This is why many people have to be admitted to intensive care in countries that have large COVID-outbreaks. However, the rather frightening statistics of intensive care admissions and deaths tend to hide an important fact: for most people, the symptoms of COVID-19 are mild, and they may even be absent all together. However, the virus does seem to hang around for quite a while in the lungs before it disappears from the body, at least in symptomatic cases, which is why symptoms and the risk of complications may persist for several weeks. Coronaviruses are among the bigger and more complicated viruses, and it seems that SARS-CoV-2 is better than influenza at suppressing parts of our immune response, which may help explain why it sticks around the body for so long. Also, the fact that the SARS brothers use the ACE2 molecule to get into cells seems to contribute to the common occurrence of pneumonia symptoms and the risk of developing ARDS.13
So what does a mild COVID-infection look and feel like, if you’re among the estimated 54-82% of people that do experience symptoms? As we’ve already established, it starts with systemic symptoms (fever, headache, etc.) in most cases, followed by something that resembles a cold in about half of the cases. During this first week you are the most infectious to other people (actually regardless of which virus you have), so keep your distance, don’t sneeze or cough without covering your mouth and wash your hands very regularly. In a little over a third of the cases you may experience mild symptoms of viral pneumonia after a few days, such as shortness of breath and pressure or pain on the chest. After about 6-12 days, your symptoms may go away, and that may be it. Or not, because in many mild cases, something strange seems to happen. This is what my symptoms looked like, more or less:
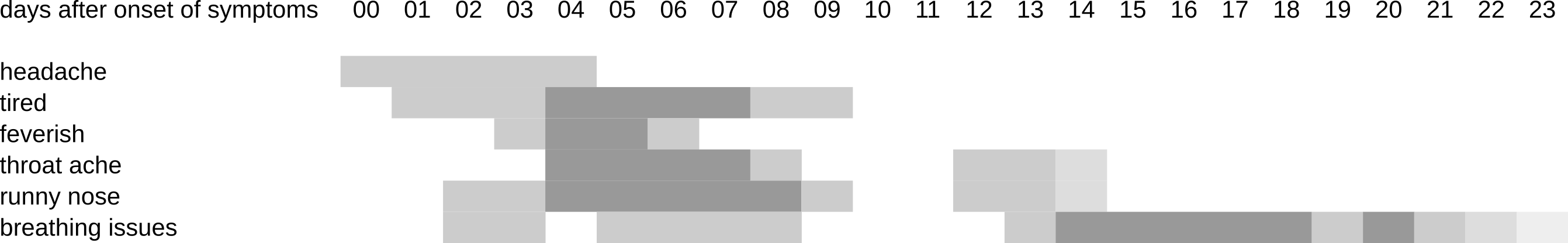
On days 2 and 3, I hiked up two mountains, which may not have been the smartest thing to do because the effort almost certainly suppressed my initial immune reaction, quite likely leading to higher virus loads later on and extending the duration of my symptoms. But despite that, my symptoms were gone after about 10 days. However, two days later, they started coming back, and eventually I had to endure another 11 days or so of shortness of breath and pressure on the chest, symptoms typically associated with mild viral pneumonia. So what happened? I certainly didn’t climb any more mountains on day 10 or 11, so something else was going on. I wrote down a more detailed analysis on this page, but the summary is that a “short break” of 1-3 days in the symptoms seems to be quite common for mild COVID-cases, and may coincide with a “dip” in the virus load around day 10-13. After that however, a relapse of symptoms may occur, and especially the symptoms of pneumonia may persist for another one or two weeks (and in some cases much longer). The little data that we have also seems to suggest that the progression of virus load in cases with symptoms of virual pneumonia looks much more like SARS, when compared with milder cases. Moreover, asymptomatic cases actually seem to have virus loads that are similar to cases with mild symptoms, although the virus is cleared by the immune system within 10-12 days or so if symptoms aren’t present.
Asymptomatic cases may contribute to spreading COVID-19 “under the radar”, although it is currently unclear how big this contribution is. For influenza it seems that there is little evidence that asymptomatic individuals play an important role in spreading the virus, so this may also be the case for COVID-19.14 However, there is still much uncertainty about that, because the evidence is rather inconclusive for influenza, and SARS-CoV-2 may be more infectious than influenza.
Actually it is somewhat of a long-standing mystery why some people experience symptoms when infected with a virus, while others do not show any symptoms. A comparison of the genes expressed in symptomatic and asymptomatic influenza infections showed that people with asymptomatic infections basically seem to “run a different genetic program” than people who experience symptoms. Asymptomatic people show a tightly coordinated immune response, while symptomatic people show the much more generic response that we know by now, which involves recognition of cell damage and bits of virus, and activation of inflammation. It is however not clear what causes this difference in the two responses, as virus load and antibody production are more or less identical in both cases. One interesting but unconfirmed possibility is that past exposure to a sufficiently similar virus may cause the immune system to recognise a new but related virus, and respond differently than if the virus were completely new.15 If this is indeed the case, asymptomatic cases of COVID-19 could be people who have recently been exposed to other human coronaviruses, such as HCoV-OC43 or HCoV-NL63, which can cause common cold. This is a testable hypothesis, and it would be interesting to see if this is indeed the case. However, it could also be that the difference in responses is caused by differences between the immune systems or immune state of individuals, or by differences in the site of infection or the initial virus load at the moment (or moments) of infection. It is even possible that asymptomatic and mild cases are people that haven’t been infected yet by similar coronaviruses, while the more serious cases have experienced prior infection. The possible mechanism behind this is known as immune priming (and/or antibody dependent enhancement), and basically involves enhanced activation of the immune system upon infection with a similar virus. While this could be a good thing, experience from past vaccine development shows that such an enhanced immune response can also lead to a stronger inflammation reaction in the lungs later on, and therefore more serious complications. A similar mechanism may also be behind the relapse of symptoms that I and others experienced in the second week of being sick.
Whatever the underlying causes may be, it seems that COVID-19 may manifest itself in several forms: without symptoms (which may be somewhat infectious), as a mild upper respiratory infection of 1-2 weeks, as an extended upper and lower respiratory infection that lasts 2-4 weeks or sometimes even longer (often with symptoms of mild pneumonia) or as a more severe pneumonia with an increased risk of complications. The risk of complications seems to be highest in the second and third week of symptoms, so it is important to know how to recognise such complications. The WHO guide for treatment of COVID-19 indicates that cases with mild symptoms of COVID-19 and/or pneumonia do not need medical treatment. But if your breathing rate exceeds 30 breaths per minute, your blood oxygen saturation drops below 93%, you feel like you’re suffocating or if you experience a sudden onset of high fever, you may be developing severe pneumonia and you need to seek immediate medical attention. If you experience shortness of breath and want to be sure, you can order an oximeter to monitor blood oxygen saturation. Oxygen saturation should normally be above 94% at sea level, although it may be somewhat lower if you live at a higher altitude. When in doubt about the severity of symptoms, it’s best to contact a doctor for medical advice, as any complications should be treated as early as possible. Also, if you have existing health problems it’s wise to get medical advice, especially if you also take medicines against high blood pressure or drugs that suppress parts of the immune system (corticosteroids, diclofenac, ibuprofen and several others).
In fact, it seems that timing is important in many ways. Your chances of surviving complications are higher if they are treated before your lung function is significantly degraded. And it seems that the fact that COVID-19 is much less deadly than SARS could be related to its tendency to infect the upper respiratory tract. While infecting the upper airways definitely helps spread the virus, it may also buy your immune system valuable time to mount an immune response. The German study of 9 patients by Wölfel et al. showed that the blood of all patients started showing antibodies against the virus 5-14 days after the onset of symptoms, and that lung virus loads remained higher for a longer period in the people who were slow in producing antibodies. In contrast, in the 75 SARS-patients studied by Peiris et al. in 2003, antibodies only showed up 11-28 days after the onset of symptoms (with an average of 20 days). It seems therefore possible that the response mounted to the relatively harmless upper respiratory infection allows our immune system to start limiting the spread of the virus in the lungs, in most cases before it can cause severe pneumonia. This would be consistent with the fact that older people, who have a slower and less effective immune response, have an increased risk of developing severe pneumonia.
Beyond the individual level, buying time is important for society as well. In the short term, lockdown and social distancing help slow the initial spread of COVID-19, and reduce the flood of severe pneumonia cases that may otherwise overwhelm the healthcare system. However, lockdown and even social distancing cannot be sustained indefinitely, if only because an increasing number of people would not accept such restrictions in the long term. Some governments are now considering a strategy of control, such as the one employed by China, South Korea and Singapore, with tracing and containment of infected cases. While this does seem successful in the short term, it is a fairly fragile strategy that is likely to break down in the longer term. You can never fully control a virus outbreak, especially since the virus has “escaped” and spread across the globe, so small (or large) outbreaks would keep popping up for years. Also, a control strategy would require that travel and public events remain restricted, probably for several years. This would destroy significant sectors of the economy and would require a large investment in terms of resources for tracing and testing, which will not be an option for many countries. On the other hand, letting the pandemic run its course, as happened with nearly every pandemic until a few decades ago, would paralyse healthcare systems and cause a significant number of deaths, especially because we’re still only at the beginning of the pandemic. Blood testing for antibodies in Austria in the first week of April suggested that less than 1% of the population had been infected so far, and around 3% of blood donors in the Netherlands tested positive for antibodies in the first weeks of April. Further preliminary results from Gangelt, a German region that experienced a relatively large outbreak, suggested that only around 15% of the population there had been infected. It seems therefore that we are still quite far from a level of “herd immunity” that would protect us from the kind of serious outbreaks that cause a rapid, exponential increase in infections, hospitalisations and deaths.
So what perspective does this give us for the longer term, if we cannot really control the virus but if we do want to prevent people ending up in hospital or dying? What we need in the long term is either a vaccine, or a way to reduce the number of complications and deaths from pneumonia, or preferably both. Of course we could get lucky, if the virus would evolve into a variant that causes mostly mild infections, similar to the other human coronaviruses that mainly cause a common cold. While viruses do generally become less dangerous in the long term, the problem is that we cannot really count on this in the short term. The virus is probably transmitted between people primarily in the first week of symptoms, while the risk of severe pneumonia mostly occurs in the second and third week. This suggests that the virus will experience little or no selection pressure against causing complications that require hospitalisation, even if the virus would have much genetic control over that (which may not be the case, as symptoms and complications are caused to a large extent by the immune response of the host).
Currently, a significant number of existing drugs is being tested, to see if any or several are effective in treating patients with COVID-19. Candidates include Favilavir, (hydro)chloroquine, Remdesivir, Lopinavir/Ritonavir, Umifenovir and a number of other drugs, as well as more generic substances such as intravenous vitamin C and interferon beta. Many of these treatments focus on slowing down or stopping the replication of the virus. However, while suppressing virus replication can indeed be effective early on in the infection, it is known from influenza that it doesn’t really help much if a patient is already at the stage of serious pneumonia symptoms. Moreover, widespread use of virus inhibitors could easily cause the virus to evolve drug resistance. So if the goal is to reduce serious complications, it may be more effective to focus on the role of the immune response itself rather than the virus. For instance, there is some evidence that treatment of COVID patients with systemic corticosteroids, which reduce inflammation, has adverse effects when used too early in the infection, but does seem to reduce symptoms and increase survival for patients once severe pneumonia sets in.16 This makes sense, as inflammation helps to control the virus early on, but may aggravate symptoms once the virus has infected the lung sacs.13 Intravenous vitamin C also targets the symptoms of viral pneumonia, as it may reduce the damage caused by reactive oxygen species that are produced by white blood cells in the lungs.7 Earlier research on influenza-induced viral pneumonia and SARS/MERS suggested several other possible treatments, although unfortunately it seems that the majority of these has not been tested yet on human patients. Also, recent research has suggested that a well-timed combination of several existing drugs could be effective in treating viral pneumonia in hospitalised COVID-patients13, although this hasn’t yet been confirmed in practice.
One treatment that is conceptually very simple and has shown some promise in treatment of 10 Chinese patients with severe cases of COVID-19 is convalescent plasma therapy. This treatment, also known as serum or antiserum therapy, has been around since the late 19th century and involves taking blood from animals or people who have already been infected by a virus, and injecting their (filtered) blood plasma into patients who are currently infected. The antibodies present in the blood plasma can help control and clean up the virus and may help bring down the symptoms of infection within a few days. Larger randomised trials are still needed to see if plasma therapy is really effective against COVID-19, but if it is, the method could be used almost immediately to treat severe cases. This could reduce the number of deaths and perhaps speed up the recovery of hospitalised patients, which would relieve some of the pressure on the healthcare system. However, because plasma therapy probably requires blood from one recovered person for each person treated, it presents a significant logistic challenge. Finding large numbers of people who have been infected and have produced sufficient antibodies and are willing to donate blood isn’t trivial, especially if the percentage of asymptomatic cases is high and testing for antibodies or infections is limited (as is currently the case in most countries). But if it is shown to be effective, plasma therapy may represent an important short-term avenue for treatment, and in the longer term other forms of passive antibody therapy may become available.
Ultimately, efforts to control COVID-19 would probably require a vaccine that would offer protection against SARS-CoV-2, but unfortunately production of such a vaccine isn’t trivial. While there are currently some 115 vaccine candidates, only five so far are ready to be tested on humans. Furthermore, it is estimated that developing and testing a safe and effective vaccine will take at least 18 months, after which it would still need to be produced and distributed in very large amounts, and applied in vaccination programs at global scale.
It is interesting that seemingly “large” problems such as poverty and inequality, climate change and the massive global loss of biodiversity and degradation of fertile soil have hardly had any impact on our behaviour, while something as tiny as a virus has caused massive behavioural change across the globe. COVID-19 has led to a complete shutdown of entire economic sectors, and has brought the mighty machine of global economic growth to a screeching halt. This is especially remarkable because virus pandemics are by no means new or uncommon, and actually occur several times per century. It seems that we are finally willing and able to let go of “business as usual”, as soon as we fear for the lives of ourselves and our loved ones. Also, COVID exposes the limitation of technological solutions when faced with something as “simple” as a virus, a small package of genetic material that isn’t even technically alive. In the short term at least, the appearance of such a new virus (with the mighty power of molecular evolution behind it, as well as the mind-boggling complexity of immune system interactions) seems well beyond our human ability to easily control with an engineering-approach, although the speed with which the scientific community has made progress in understanding the virus is truly impressive. In itself a pandemic has rarely been a huge problem for humanity, although it has been for individual humans. Disease and death have always been a feature of life, and actually our immune system is remarkably successful at limiting the damage in most cases. However, a pandemic such as the current one does become a serious problem as soon as we stop accepting risk, disease and death as facts of life, and if we (quite understandably) want to minimise the number of deaths, while at the same time wanting to avoid paralysing social life and large parts of the economy. Welcome to modern life. It seems a crisis such as this exposes both the fragility and the strengths of our social and economic systems, and probably this is not such a bad thing…
It seems that, until some kind of treatment becomes available, our best protection is still good health and a strong immune system. In a world where around a quarter of all people suffer from metabolic syndrome, and in which stress and lack of sleep are endemic, this may be a problem. Probably the best advice I can give is this: Don’t do what Boris Johnson did. The British Prime Minister contracted COVID-19, but rather than taking a rest he continued to (quite literally) almost work himself to death. Here is a better approach: as soon as you experience any symptoms of upper respiratory disease, you should stop working, avoid stress or heavy physical exertion and make sure you get plenty of sleep. It seems likely that the initial immune response to COVID-19 is very important in determining the risk of complications, and getting sufficient sleep has been shown to be important for a healthy immune response. Among other negative effects, sleep deprivation decreases the production of both cytokines and antibodies, so lack of sleep reduces the ability of your body to mount an effective immune response. Stress has also been shown to impair immune system functioning, especially as people get older. So your best protection against COVID for now may be to get sufficient sleep and take it easy. And in order to protect others, especially ones who have a higher risk of complications, stay away from them as soon as you experience any symptoms, especially during the first 8 days, but preferably at least as long as symptoms persist. Moreover, if you experience symptoms, stay away from work and enclosed spaces with other people and cover your mouth and nose. It also helps to ventilate spaces well, so open the windows if temperature permits. Like influenza, COVID-19 probably spreads mostly through small droplets that infected people produce when sneezing, coughing or even talking.3 In spaces that are not well-ventilated, especially if many people are present, these virus-laden droplets can hang around for quite a while and easily infect others, especially if humidity is low (below 45%) or somewhat high (around 65%).17
COVID-19 is a disease that travels easily, and has profited immensely from a globalised world with ubiquitous cheap flights. I most likely contracted the disease in a hostel, and I was sick for three weeks, having to avoid contact with others in a situation in which that was not so easy. Like most people of my age group (and younger), I eventually recovered without further complications. In fact, the people around me who most likely also had the virus, experienced only a few days of discomfort. My symptoms were probably aggravated by my unfortunate choice to hike up mountains in the early days of the infection. I realise that I am in quite a privileged position, in quite a few ways. Of course I do worry about loved ones and I miss hugs and friends and social events. But at least I may have experienced COVID-19 already, I did not run much of a risk, I am still healthy and at least in the short term I still have a job and an income. Many people are not so fortunate, and I wish all of you good luck in these strange times. Until some kind of progress is made in resolving this COVID crisis, I mostly try to enjoy the odd departure from normality that this pandemic represents. I try to make time to read and reflect, sit in the sun and spend time by myself just enjoying my surroundings. Above all, I’m very curious to see how this all plays out. I cannot predict the future, and neither can anyone else (although everybody seems to try). But I do know that, sometimes, good things can come from a crisis. Let’s see. These certainly are interesting times.
COVID-19 generally starts off as an upper respiratory infection, similar to common cold and flu. The typical symptoms of such an infection are actually not caused by the virus but by your immune response, which is why they don’t say much about which virus is the culprit. If the cells lining your upper airways are infected by a virus, your immune system often responds by producing molecules called cytokines. These cytokines may initially trigger a number of systemic symptoms, such as headache, fever, muscle ache, loss of appetite and/or a general feeling of being sick. Somewhat later, cytokines trigger localised inflammation responses, which are responsible for symptoms typically associated with cold or flu, such as a sore throat, a runny nose and sneezing and coughing. Unfortunately some of these responses also contribute to spreading the virus to other people. Small airborne water droplets are produced by people who sneeze, cough or even breathe. Especially at the peak of upper respiratory infection symptoms (the first week of infection), such droplets may contain significant amounts of virus, which can be inhaled by other people and lead to infection.
While a cold mostly remains restricted to the upper respiratory tract, flu and COVID can move down into the lungs and cause viral pneumonia, an inflammation of the lung tissue. If the infection reaches the alveoli of the lungs, the resulting tissue damage and immune reaction may impair the uptake of oxygen. Symptoms of mild viral pneumonia seem common in a little over a third of symptomatic COVID-cases and may include shortness of breath and pressure or pain on the chest. COVID-19 may manifest itself in several forms: without symptoms (which may be somewhat infectious), as a mild upper respiratory infection of 1-2 weeks, as an extended upper and lower respiratory infection that lasts 2-4 weeks (often with symptoms of mild pneumonia) or as a more severe pneumonia with a higher risk of complications. Serious respiratory distress (e.g. more than 30 breaths per minute) may indicate severe pneumonia, in which case you should seek immediate medical attention.
Data from both COVID and flu infections suggest that asymptomatic cases of COVID-19 may be common, have a similar virus load but are less prolonged and probably involve a different immune response “program”. What causes this is not known, although it may involve cross-immunity caused by other coronavirus-infections, differences between the state of people’s immune systems or differences in the amount of virus people are exposed to.
Prolonged disease and increased risk of complications may be the result of a weak or slow initial immune response to the infection. This may allow the virus to eventually spread further in the lungs, and trigger a stronger inflammation reaction associated with pneumonia in the second or third week of the infection. To avoid this, it is important to avoid sleep deprivation, stress and heavy physical exercise early in the infection, as these may interfere with the immune response. Moreover, stay away from others (especially sick and elderly people) if you experience any symptoms, and cover your face if you do need to be near other people, as the risk of infecting others is probably highest in the first 8 days of symptoms. Infection risk is especially high in spaces that are not well ventilated and that have dry air (relative humidity below ca. 45%) or relatively humid air (around 65% relative humidity), because droplets with virus particles may remain present in the air for longer periods under such conditions.
Footnotes and further details:
-
Another 10-15% of cases of common cold are caused by human coronaviruses, which have rather obscure names such as HCoV-OC43, and some of which have been circulating in the human population for at least half a century, although probably much longer.
See Lia van der Hoek (2007), “Human coronaviruses: what do they cause?” ↩ -
So what happens if you get a cold? First, you get infected by “live” virus particles, also know as virions
Because a virus isn’t technically alive, a “live” virus particle in this case means one that is still infectious, that is, able to successfully infect a host cell. ↩ -
either because you inhale tiny droplets that other people produce when sneezing or coughing, or because you pick them up from a surface (or person) that was contaminated with live virions. […] Like influenza, COVID-19 probably spreads mostly through small droplets that infected people produce when sneezing, coughing or even breathing.
It is still not entirely clear what the most important infection route is, and it probably depends on the virus as well. While virus RNA is found on many “infected” surfaces, it seems rare to actually find infectious virions on such surfaces, at least for coronaviruses. It is believed that for most upper respiratory infections, the main route of infection “at a distance” is through airborne droplets, especially the relatively large droplets that are spread when people cough or sneeze without covering their mouth. These can be inhaled by people who are nearby, and infect the mucous membrane of the respiratory tract or even the eyes. This can happen indirectly as well, if someone sneezes into their hands and then touches another person, or an object that is subsequently touched by another person, and this other person then touches their eyes, nose or mouth. However, because indirect routes will probably yield lower numbers of infectious virions, and virions do not survive for long outside the body, the probability of indirect infections is almost certainly lower. Also, while small sneeze droplets can travel up to 7-8 m, they contain less virions than larger droplets. Larger droplets travel much less far, so that the probability of direct infection will probably decrease rapidly with distance. Much is still unknown, but in any case sneezing into your elbow and/or wearing some kind of face protection is expected to significantly reduce the risk of direct infection of other people through airborne droplets, so this is a good basic precaution, although it is certainly not guaranteed to stop spread of a respiratory virus. And especially between partners and children, viruses are probably more likely to spread through direct and indirect contact. ↩↩ -
As a result of the typical properties of flu viruses, an influenza infection may evoke a stronger immune response than a common cold infection, and has a larger probability of causing complications.
To slightly confuse things, common cold is actually caused by influenza viruses in roughly 5-10% or cases, while some instances of common cold can develop into a serious lower respiratory infection. But in this article I will use the term common cold for a relatively mild upper respiratory infection. ↩ -
The first “local” (rather than systemic) symptom is often irritation of the throat or nasal cavity, which may later develop into a sore throat as inflammation gets worse. Sneezing is another local effect of inflammation, often accompanied by a runny nose. Unfortunately both these responses of your immune system, sneezing and a runny nose, also happen to be great ways of spreading virus particles so that they can infect other people. As the inflammation spreads down in the direction of the lower respiratory tract, it may over-sensitize the nerves that trigger the coughing response, which may then cause you to start coughing at even the slightest irritation of the airways (such as inhaling cold air).
See Eccles (2005) for a detailed description of common cold and flu symptoms and their origin. ↩ -
For instance, fever is uncommon with common cold in adults, but it is reported in roughly 35% of experimental infections with influenza (in adults younger than 65).
See Carrat et al. (2008) for a meta-study of experimental influenza infection results in adults. ↩ -
The resulting influenza strains were new to our immune systems, and so spread rapidly in the human population and caused more damage than usual.
The “novelty” of a virus strain seems to be important for it to become a pandemic, but its pathogenicity (the degree to which a virus is dangerous) may be determined by other factors as well. Brandes et al. (2014) studied the difference between two influenza strains, one that produced mild symptoms and one that was highly pathogenic, generally caused lethal pneumonia and is thought to be similar to the strain that caused the 1918 pandemic. They showed that the highly pathogenic virus strain overwhelms the innate immune response (your body’s “first line of defense”) early on in the infection. And while the virus load of both strains was similar, the highly pathogenic strain managed to spread much more widely across the lungs. Because the highly pathogenic strain infects more of the lung tissue, it triggers a stronger response from neutrophils (a type of white blood cells), which then cause more lung damage, eventually leading to death of the host through oxygen deprivation. So virus load by itself isn’t necessarily a good predictor of complication risk, the size of the infected area is more important for lower respiratory infections. In COVID-19 something similar may be going on: a weak immune response early in the infection may cause a larger lung area to be infected, which subsequently triggers a strong local immune response that causes further lung damage. While studies of the immune responses involved in SARS and MERS suggest that the details differ between influenza and SARS-like coronaviruses, the general mechanism seems to be the same. In both cases, the failure of early immune containment may lead to a hyper-inflammatory response in the lungs later in the infection. In the case of the coronaviruses (but possibly also in influenza), active suppression of the early immune response by the virus may aggrevate this problem. ↩ -
So the first symptoms of COVID-19 you’d experience are the systemic ones (fever, headache, muscle ache, loss of appetite, loss of smell and/or taste, feeling tired, generally feeling sick)
The loss of smell and/or taste seems to be uncommon in viral infections, but has been reported by a number of COVID-19 patients. It is believed to result from infection or inflammation of nerve tissue, although the precise cause is currently unclear. While ACE2 is present in the central nervous system, it is not generally present in nerve tissue, so infection of nerve cells seems unlikely. ↩ -
In the second week of March, two hospitals in The Netherlands tested 1353 health care workers with symptoms of respiratory infection, and found 86 (6.4%) to be infected with SARS-CoV-2.
See Kluytmans et al. (2020) for a description of this study and its results. ↩ -
let’s take a look at how the virus load (the amount of virus in the body) develops after the onset of symptoms, for influenza, COVID-19 and SARS
The influenza virus loads in the figure (right axis) are averages reported by a literature study of infection experiments by Carrat et al. (2008). I assumed here that day 1 post-infection is the first day of symptoms. The COVID-19 virus loads are taken from Wölfel et al. (2020) and are the daily averages of the virus loads reported for 8 patients with mild symptoms and 1 patient with no symptoms. The SARS virus loads are the averages reported by Peiris et al. (2003) for day 5, 10 and 15 after the onset of symptoms. Both scales are log10 virus loads, which means that they are logarithmic: 2 corresponds to 100, 3 corresponds to 1000, 6 corresponds to a million, etc. Values below 2 (100) may not be accurate, at least for the COVID-19 virus loads. For the latter, the numbers are log10 RNA copies per swab for throat swabs, and log10 RNA copies per mL for the lung samples (which are sputum samples). Care should be taken in interpreting the values plotted here, because methods, measures and number of patients vary between studies. The purpose of this figure is to compare the timing of virus load peaks, rather than to compare the actual viral loads. ↩ -
COVID-19 is reported to have an average incubation period of 2-6 days, with much longer incubation times (up to two weeks) reported for some cases.
See Rabi et al. (2020) for an overview of what we knew about COVID-19 based on the early outbreaks in Asia. ↩ -
Also, the fact that the SARS brothers use the ACE2 molecule to get into cells seems to contribute to the common occurrence of pneumonia symptoms and the risk of developing ARDS.
In a recent paper, Van de Veerdonk et al. proposed a mechanism to explain why SARS and COVID-19 so often cause mild and/or serious symptoms of pneumonia. The two coronaviruses bind to ACE2, a molecule on the outside of certain types of cells that, among other things, is involved in regulating blood pressure and parts of the immune system. The latter function involves controlling the effects of bradykinin, a messenger molecule that is activated by cytokines and helps induce local inflammation. The problem is that by interfering with the role of ACE2, the viruses may also increase the inflammatory effects triggered by bradykinin. In the lungs, this local inflammation may cause the tissue to become “leaky”, so that the air sacs of the lungs start filling with fluid. This fluid reduces the uptake of oxygen in the lungs, causing shortness of breath and/or a feeling of pressure or pain on the chest. In hospitalised patients with COVID-19, breathing problems often get worse around day 9 of symptoms. This could actually be caused by the production of antibodies against the virus around this time. Antibodies that reach infected parts of the lungs, bind to the virions there and attract immune cells that may cause further damage to the lung tissue, an effect known as antibody-dependent enhancement (ADE) of lung damage. A similar effect has also been described for influenza, but it may be worse in SARS and COVID-19 because the problems with ACE2 cause the inflammation reaction to be stronger. This effect may be even worse for people that take drugs against high blood pressure, as these often suppress the function of ACE2. The researchers propose that for serious cases of COVID-19, a well-timed combination of antiviral drugs, generic anti-inflammatory treatment and specific drugs that block the deleterious effect of bradykinin may prevent progression of symptoms to the point where intensive care treatment and mechanical ventilation are needed. ↩↩↩ -
For influenza it seems that there is little evidence that asymptomatic individuals play an important role in spreading the virus, so this may also be the case for COVID-19.
See Patrozou & Mermel (2009) for a literature study on the possible role of presymptomatic and asymptomatic individuals in spreading influenza. COVID infection experiments using rhesus macaques show that these monkeys exhibit similar symptoms to humans, and suggest that the timing of their symptoms matches pretty well with the virus load in various parts of the airways. Similar experiments on cynomolgus macaques however resulted in mostly asymptomatic infections, with detectable virus loads in the nose and throat for several days, although the virus was seemingly cleared somewhat faster than in humans (especially in young animals). Supplementary table S1 of the latter study shows that infectious virus could be isolated for 4 days from the nose and/or throat of these monkeys. An earlier study on the transmission of influenza between ferrets suggests that influenza is mostly transmitted through airborne droplets generated during upper respiratory infection of the nasal cavity. However, in the absence of symptoms such as sneezing, coughing and a runny nose, it is unclear how efficiently the virus could spread to other individuals. ↩ -
One interesting but unconfirmed possibility is that past exposure to a sufficiently similar virus may cause the immune system to recognise a new but related virus, and respond differently than if the virus were completely new.
This effect is called cross-immunity. In humans there have been indications that a certain degree of so-called heterosubtypic cross-immunity exists for influenza viruses, which may be related to the observed high percentage of asymptomatic infections. For instance, Meade at al. (2020) describe some degree of apparent cross-immunity in adults infected with influenza, but unfortunately they only included one of several asymptomatic cases. Wölfel et al. (2020) showed some degree of cross-reactivity between the antibodies against SARS-CoV-2 and other human coronaviruses, notably HCoV-OC43, HCoV-HKU1 and HCoV-NL63. ↩ -
there is some evidence that treatment of COVID patients with systemic corticosteroids, which reduce inflammation, has adverse effects when used too early in the infection, but does seem to reduce symptoms and increase survival for patients once severe pneumonia sets in.
A review by Russell et al. concluded that evidence is somewhat mixed and at this moment inconclusive, so it currently recommends caution in the use of NSAIDs (such as Ibuprofen and Diclofenac) and corticosteroids in COVID-19 patients. ↩ -
In spaces that are not well-ventilated, especially if many people are present, these virus-laden droplets can hang around for quite a while and easily infect others if humidity is low (below 45%) or somewhat high (around 65%).
Lowen et al. (2007) studied the transmission of influenza virus between guinea pigs. Their results suggest that virus transmission through tiny airborne droplets happens most efficiently at low temperatures and low humidity. However, at room temperature, transmission efficiency showed two peaks: one at lower air humidity, below ca. 45%, and one at higher humidity, around 65%, as shown in Figure 6 from Lowen et al. (2007):
It is believed that the stability of influenza virions (and probably also coronaviruses) in droplets is highest around low (20%–40%) and high (60%–80%) relative humidity, and minimal at intermediate humidity (50%). But at very high humidity (around 80% or more), the droplets tend to take up water from the air, grow in size and settle more rapidly. Conversely, at low humidity, larger droplets (with more virions) evaporate water, decrease in size and stay airborne longer. Moreover, smaller droplets can also be inhaled more easily and may end up deeper in the respiratory tract.
Dutch pollster Maurice de Hond has analysed the geographical distribution of large outbreaks of COVID-19, and suggested that there is a fairly robust relationship with relative humidity, the degree to which people spend time in badly ventilated spaces (high in areas that are cold and/or relatively wealthy, often lower in areas that are warm and/or relatively poor) and the occurrence of large gatherings such as (religious or non-religious) festivals. Indeed, data from the distribution of influenza and RSV (Respiratory Syncytial Virus) outbreaks seems to support this analysis. If this is indeed the case, we could expect that the incidence of large COVID-outbreaks may go down significantly in summer in temperate regions, and may go up slightly in the wet season in more tropical regions, as shown for influenza in figure 3 of Bloom-Feshbach et al. (2013):
The results above also suggest that it may help to keep spaces such as offices, stores, restaurants, cafes, classrooms and homes well ventilated and (if possible) the air at around 50% relative humidity, to spend more time outdoors and to limit or avoid large gatherings (especially indoors) as long as the pandemic isn’t under control. ↩